Bakalářské práce/Bachelor’s Theses
Diplomové práce/Master's Theses
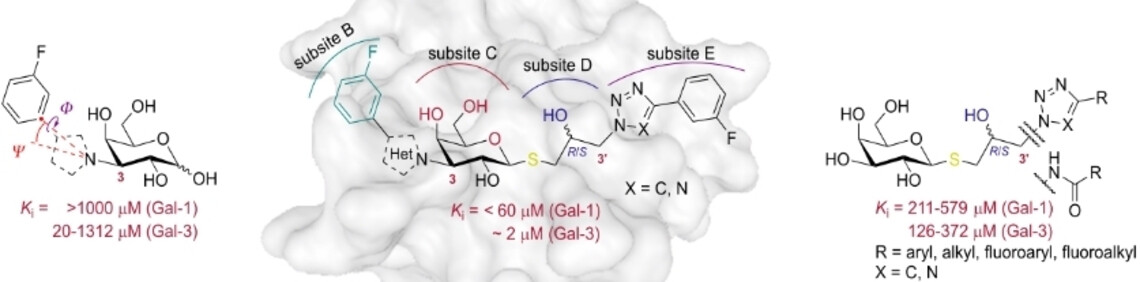
33. Zýka. J.; Kozák, J.; Vanekova, L.; Polidarova, P. M.; Prouza, V.; Habanová, N.; Strmeň, T.; Zavřel, M.; Pachl P.; Choutka, J.; Saskova, G. K.; Brazdova, A.; Parkan, K.; Pohl R. N-Aryl-N-Lactosylamides as Potent and Highly Selective Galectin-3 Inhibitors. J. Med. Chem. 2025, 68, 22, 24624–24648. DOI: https://doi.org/10.1021/acs.jmedchem.5c02604

32. Habanová, N.; Kaminský, J.; Parkan, K.; Zýka, J.; Prouza, V.; Klepetárová, B.; Pohl, R. Redundant ¹⁵N-Mediated J-Couplings Reveal an Aglycone Conformation in N-Phenyl Glycosylamines. J. Org. Chem. 2025, 90, 45, 16047–16059. DOI: https://doi.org/10.1021/acs.joc.5c01892.

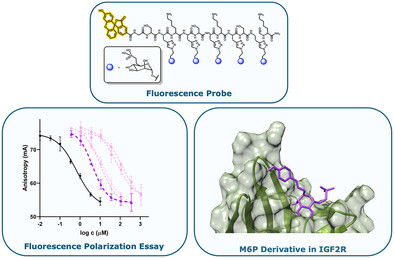
31. Mrázková, L.; Hladoníková, K.; Toncarová, B.; Fischer, M.; Zýka, J.; Kozák, J.; Král, M.; Kožíšek, M.; Jiráček, J.; Kaminský, J.; Parkan, K.; Žáková, L. Design of Potent Mannose-6-Phosphate Derivatives as Ligands for CI-M6P/IGF2R Using Fluorescence Polarization Assay. Chem. Eur. J. 2025, 31(41), e202500973.
DOI: https://doi.org/10.1002/chem.202500973
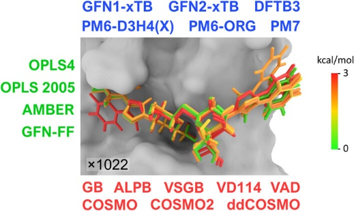
30. Choutka, J.; Kaminský, J.; Wang, E.; Parkan, K.; Pohl, R.: End-Point Affinity Estimation of Galectin Ligands by Classical and Semiempirical Quantum Mechanical Potentials. J. Chem. Inf. Model. 2025, 65(2), 762–777.
DOI: https://doi.org/10.1021/acs.jcim.4c01659
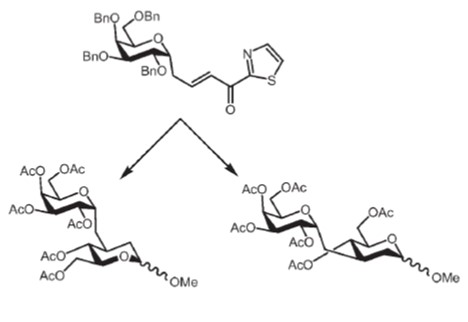
29. Prouza, V.; Zýka, J.; Kozák, J.; Magdolenová, A.; Pohl, R.; Parkan, K.: The evaluation of glyceryl C3-azolyl-thiogalactosides as galectin-1 and galectin-3 ligands. ChemMedChem 2025, e202400826.
DOI: https://doi.org/10.1002/cmdc.202400826
28. Hamala, V.; Kurfiřt M.; Červenková Šťastná, L.; Hujerová, H.; Bernášková, J.; Parkan, K.; Kaminský, J.; Habanová, N.; Kozák, J.; Magdolenová, A.; Zavřel, M.; Staroňová, T.; Ostatná, V.; Žaloudková, L.; Daňhel, D.a Holčáková, J.; Voňka, P.; Hrstka, R.; Karban, J.: Ferrocene- and ruthenium arene-containing glycomimetics as selective inhibitors of human galectin-1 and -3. Inorg. Chem. Front. 2024, 11, 7588.
DOI: https://doi.org/10.1039/D4QI01555J

27. Zýka, J.; Prouza, V.; Habanová, N.; Pohl, R.; Parkan, K.: The synthesis and characterization of electron-poor glycosylamines and derived glycosylamides. Carbohydr. Res. 2024, 536, 109023.
DOI: https://doi.org/10.1016/j.carres.2024.109023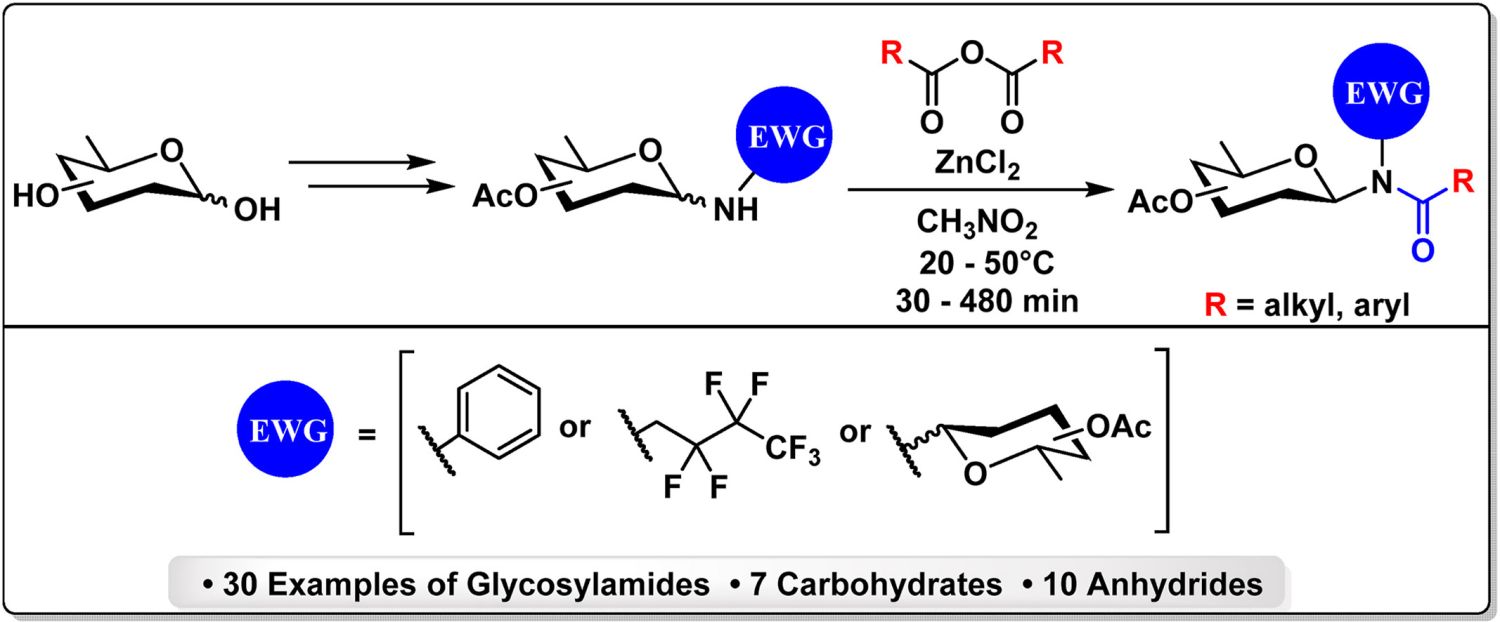
26. Choutka, J.; Parkan, K.; Pohl, R.; Kaminský, J.: On the origin of the electronic and magnetic circular dichroism of naphthyl C-glycosides: Anomeric configuration. Carbohydr. Res. 2024, 535, 109021.
DOI: https://doi.org/10.1016/j.carres.2023.109021
25. Kovalová, A.; Prouza, V.; Zavřel, M.; Hájek, M.; Dzijak, R.; Magdolenová, A.; Pohl, R.; Voburka, Z.; Parkan, K.; Vrábel, M.: Selection of galectin-binding ligands from synthetic glycopeptide libraries. ChemPlusChem. 2024, 89(7), e202300567.
DOI: doi.org/10.1002/cplu.202300567 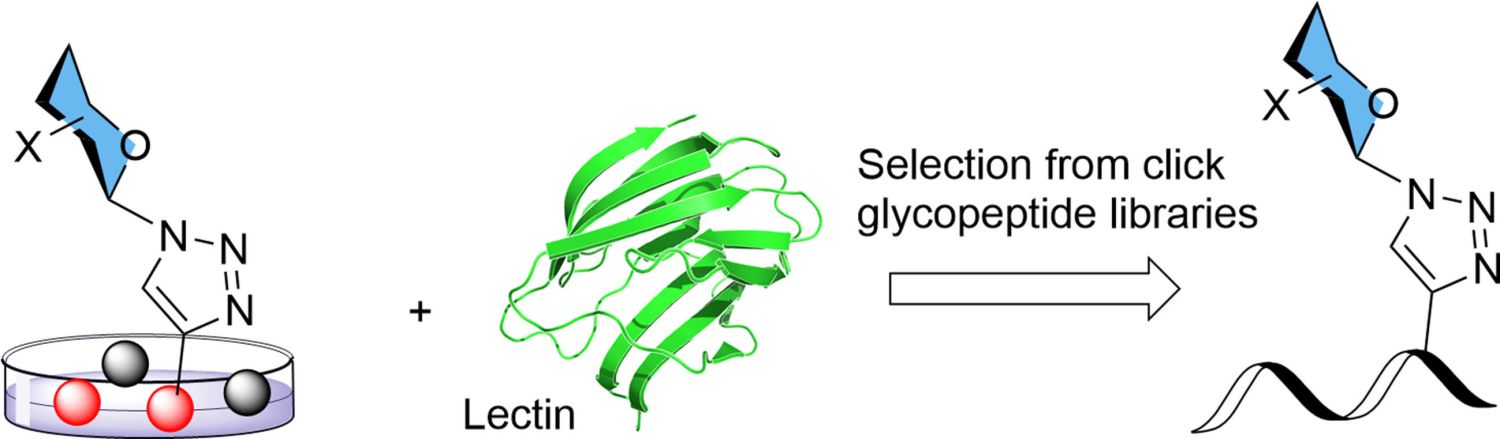
Kratochvíl, M.; Cisarová, I.; Pohl, R.; Kaminský, J; Parkan, K.: Silicon-bridged (1 -> 1)-disaccharides: an umpoled glycomimetic scaffold. Org. Biomol. Chem. 2022, 20, 7613-7621.
DOI: https://doi.org/10.1039/D2OB01161A
; Kaminský, J.; Pohl, R.; Miyazaki, T.: Unlocking the hydrolytic mechanism of GH92 alpha-1,2-mannosidases: Computation inspires the use of C-glycosides as Michaelis complex mimics. Chem. Eur. J. 2022, 28, e202200148.
DOI: https://doi.org/10.1002/chem.202200148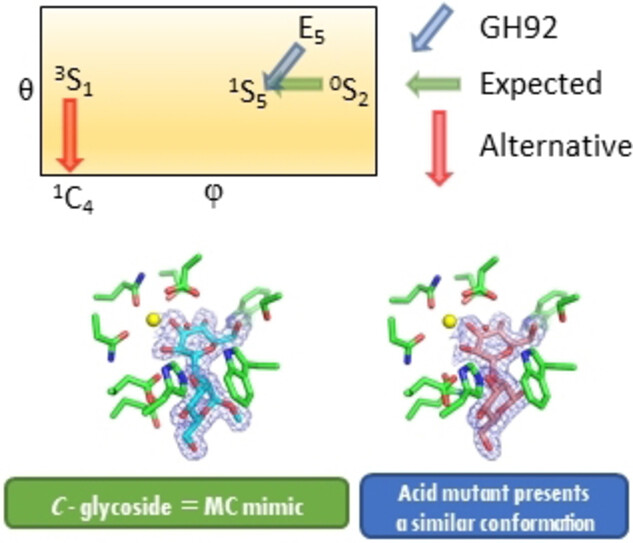
22. Vaňková, K.; Rahm, M.; Choutka, J.; Pohl, R.; Parkan, K.: Facile Approach to C-glucosides by Using a Protecting-Group-Free Hiyama Cross-Coupling Reaction: High-Yielding Dapagliflozin Synthesis. Chem. Eur. J. 2021, 27, 10583– 10588. VIP
DOI: https://doi.org/10.1002/chem.202101052
21. Vaňková, K., Šimák, O., Kettelhoit, K., Parkan, K.: One Step Transformation of Glycals into 1-Iodo Glycals. In Carbohydrate Chemistry: Proven Synthetic Methods, Vol 5. Kosma P., Wrodnigg T. M., Stutz A., CRC Press, Taylor & Francis Group:Boca Raton, 2021, Vol. 5, pp 11-16. DOI: 10.1201/9781351256087, ISBN 9780815367888. Link
20. Choutka, J.; Kratochvíl, M.; Zýka, J.; Pohl, R.; Parkan, K.: Straightforward synthesis of protected 2-hydroxyglycals by chlorination-dehydrochlorination of carbohydrate hemiacetals. Carbohydr. Res. 2020, 496, 108086.
DOI: https://doi.org/10.1016/j.carres.2020.108086
19. Faltinek, L.; Fujdiarová, E.; Melicher, F.; Houser, J.; Kašáková, M.; Kondakov, N.; Kononov, L.; Parkan, K.; Vidal, S.; Wimmerová, M.: Lectin PLL3, a novel monomeric member of the seven-bladed β-propeller lectin family. Molecules 2019, 24, 4540.
DOI: https://doi.org/10.3390/molecules24244540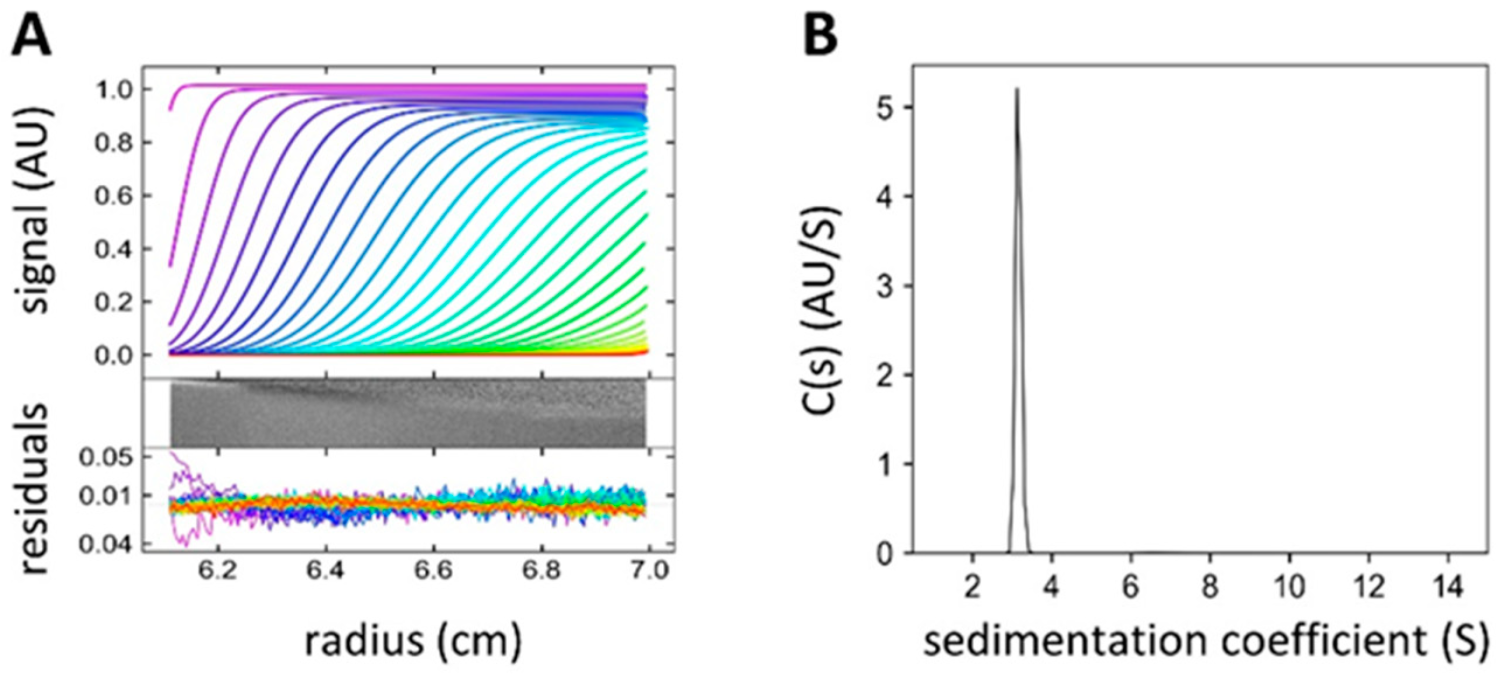
18. Jančaříková, G.; Houser, J.; Kašáková, M.; Oroszová, B.; Bertolotti, B.; Parkan, K.; Moravcová, J.; Wimmerová, M.: Fucosylated inhibitors of recently identified bangle lectin from Photorhabdus asymbiotica. Sci. Rep. 2019, 9, 14904.
DOI: https://doi.org/10.1038/s41598-019-51357-9 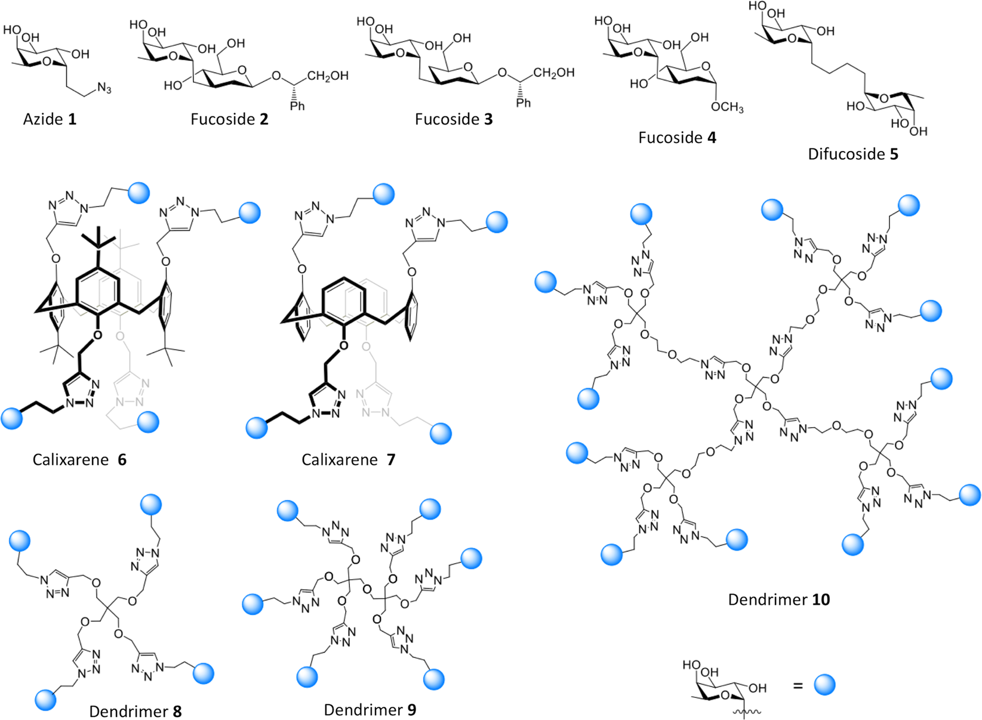
17. Choutka, J., Pohl, R., Parkan, K.: MOP and EE protecting groups in synthesis of α- or β-aryl-C-glycosides from glycals. ACS Omega 2018, 3, 7875-7887.
DOI: 10.1021/acsomega.8b00901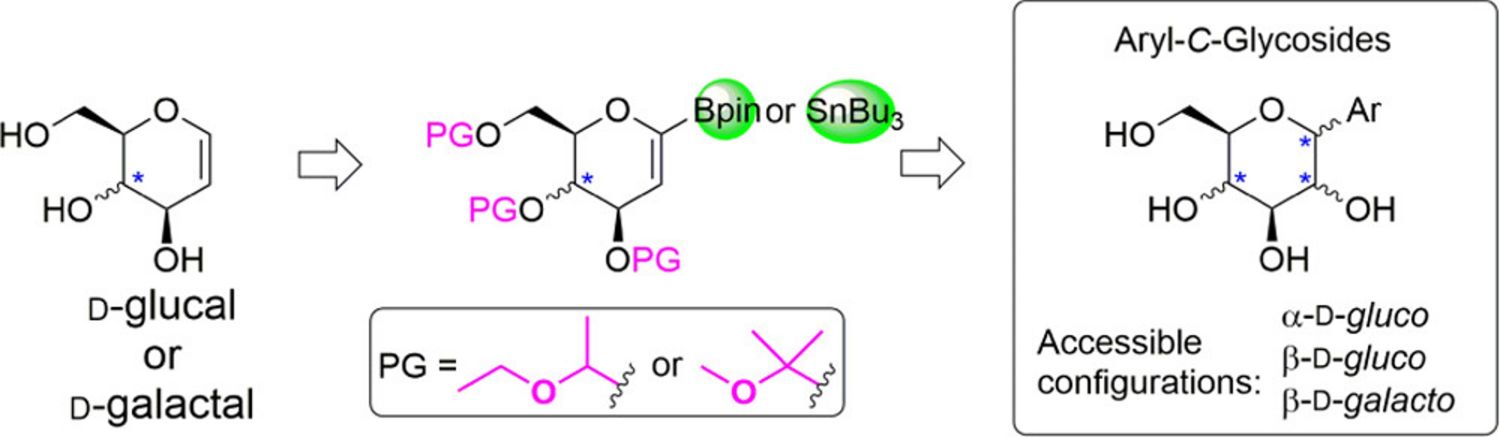
16. Raich, I.; Lövyová, Z.; Trnka, L.; Parkan, K.; Kessler, J.; Pohl, R.; Kaminský, J.: Limitations in the description of conformational preferences of C-disaccharides: The (1 → 3)-C-mannobiose case. Carbohydr. Res. 2017, 451, 42-50.
DOI: https://doi.org/10.1016/j.carres.2017.09.006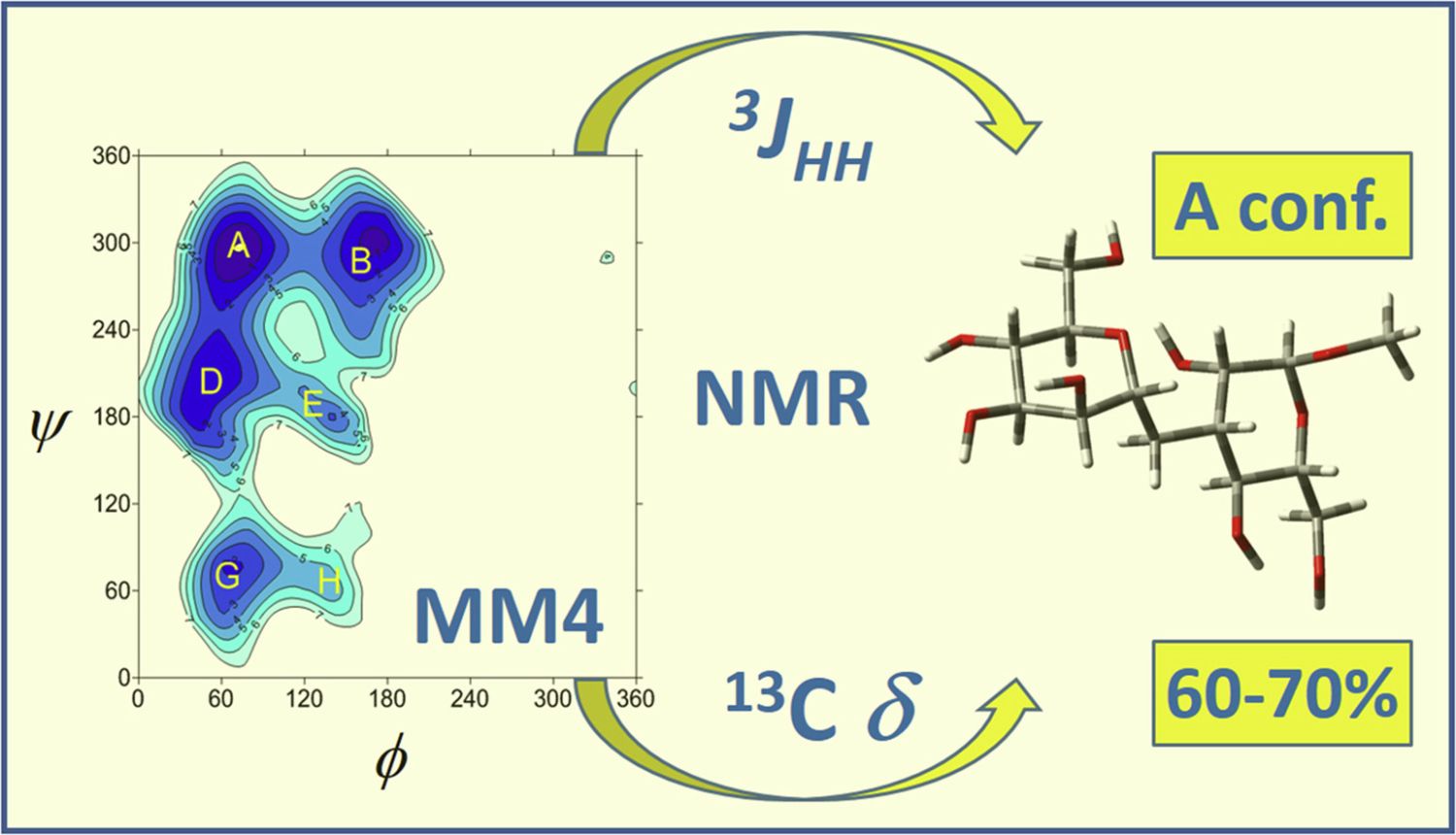
15. olyvalent C-glycomimetics based on L-fucose or D-mannose as potent DC-SIGN antagonists. Org. Biomol. Chem. 2017, 15, 3995-4004.
DOI: https://doi.org/10.1039/C7OB00322F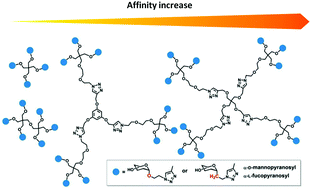
14. Bertolotti, B.; Oroszová, B.; Sutkeviciute, I.; Kniežo, L.; Fieschi, F.; Parkan, K.; Lövyová, Z.; Kašáková, M.; Moravcová, J.: Nonhydrolyzable C-disaccharides, a new class of DC-SIGN ligands. Carbohydr. Res. 2016, 435, 7-18.
DOI: https://doi.org/10.1016/j.carres.2016.09.005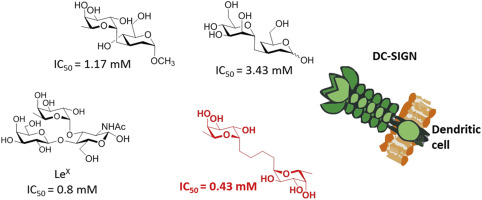
13. Kožíšek, M.; Štěpánek, O.; Parkan, K.; Albiñana, B. C.; Pávová, M.; Weber, J.;a, Hans-Georg Krӓusslich, H.-G.; Konvalinka, J.; Machara, A.: Synthesis and evaluation of 2-pyridinylpyrimidines as inhibitors of HIV-1 structural protein assembly. Bioorg. Med. Chem. Lett. 2016, 26 , 3487–3490.
DOI: https://doi.org/10.1016/j.bmcl.2016.06.039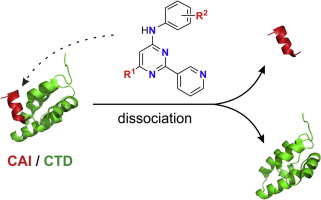 12. Machara, A.; Lux, V.; Kožíšek, M.; Grantz, Šašková, K.; Štěpánek, O.; Kotora, M.; Parkan, K.; Pávová, M.; Glass, B.; Sehr, P.; Lewis, J.; Mueller, B.; Kraeusslich, H.-G.; Konvalinka J.: Specific inhibitors of HIV capsid assembly binding to the C-terminal domain of the capsid protein: evaluation of 2-arylquinazolines as potential virostatics. J. Med. Chem. 2016, 59, 545-558.
12. Machara, A.; Lux, V.; Kožíšek, M.; Grantz, Šašková, K.; Štěpánek, O.; Kotora, M.; Parkan, K.; Pávová, M.; Glass, B.; Sehr, P.; Lewis, J.; Mueller, B.; Kraeusslich, H.-G.; Konvalinka J.: Specific inhibitors of HIV capsid assembly binding to the C-terminal domain of the capsid protein: evaluation of 2-arylquinazolines as potential virostatics. J. Med. Chem. 2016, 59, 545-558.
DOI: https://doi.org/10.1021/acs.bioconjchem.0c00706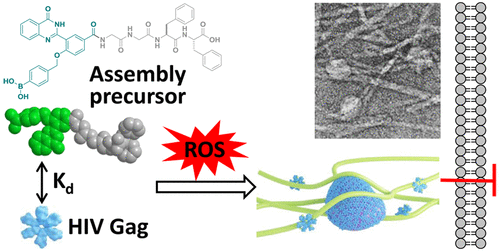
11. Šnajdr, I.; Parkan, K.; Hessler, F.; Kotora M.: Cross-metathesis reaction of α- and β-vinyl C-glycosides with alkenes. Beilstein J. Org. Chem. 2015, 11, 1392–1397.
DOI: https://doi.org/10.3762/bjoc.11.150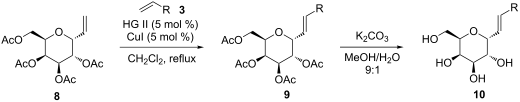
10. Oroszová, B.; Choutka, J.; Pohl, R.; Parkan, K.: Modular stereoselective synthesis of (1→2)-C-glycosides based on the sp2–sp3 Suzuki–Miyaura reaction. Chem. Eur. J. 2015, 21, 7043-7047.
DOI: https://doi.org/10.1002/chem.201406591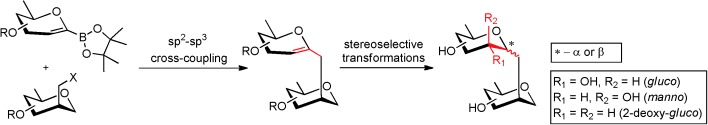
9. Kraeusslich, H.; Sticht, J.; Wildova, M.; Lux, V.; Konvalinka, J.; Kotora, M.; Grantz, Saskova, K.; Kozisek, M.; Stepanek, O.; Parkan, K.; Machara, A.: Use of substituted fused heterocyclic compounds for inhibiting HIV capsid assembly to treat HIV. WO 2014128206-A1, Aug 28, 2014. Link
8. Kraeusslich, H.; Sticht, J.; Wildova, M.; Lux, V.; Konvalinka, J.; Kotora, M.; Grantz, Saskova, K.; Kozisek, M.; Stepanek, O.; Parkan, K.; Machara, A. 2,4-Substituted or 4,6-substituted pyrimidine compound useful for inhibiting HIV capsid assembly for treating a patient suffering from HIV. WO 2014128213-A1, Aug 28, 2014. Link
7. Kraeusslich, H.; Sticht, J.; Wildova, M.; Lux, V.; Konvalinka, J.; Kotora, M.; Grantz, Saskova, K.; Kozisek, M.; Stepanek, O.; Parkan, K.; Machara, A. 2,4-Substituted or 4,6-substituted pyrimidine compound useful for inhibiting HIV capsid assembly for treating a patient suffering from HIV. EP 2769722-A1, Aug 27, 2014. Link
6. Kraeusslich, H.; Sticht, J.; Wildova, M.; Lux, V.; Konvalinka, J.; Kotora, M.; Grantz, Saskova, K.; Kozisek, M.; Stepanek, O.; Parkan, K.; Machara, A. Use of substituted fused heterocyclic compounds for inhibiting HIV capsid assembly to treat HIV. EP 2769723-A1, Aug 27, 2014. Link
5. Parkan, K.; Pohl, R.; Kotora, M.: Cross-coupling reaction of saccharide-based alkenyl boronic acids with aryl halides: The synthesis of bergenin. Chem. Eur. J. 2014, 20, 4414–4419.
DOI: https://doi.org/10.1002/chem.201304304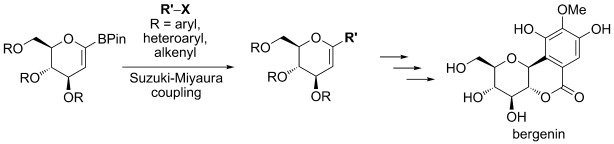
4. Lövyová, Z.; Parkan, K.; Kniežo, L.: Stereoselective preparation of four 3-C-mannosylated D- and L-glucals from a single starting compound. Tetrahedron 2011, 67, 4967–4979.
DOI: https://doi.org/10.1016/j.tet.2011.04.044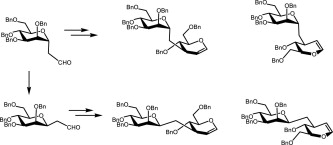
3. Olive, A.; Parkan, K.; Givelet, C.; Michl, J.: Covalent stabilization: A sturdy Molecular square from reversible metal-ion-directed self-assembly. J. Am. Chem. Soc. 2011, 133, 20108–20111.
DOI: https://doi.org/10.1021/ja209051t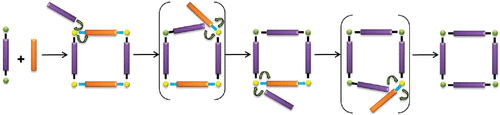
2. Parkan, K.; Werner, L.; Lövyová, Z.; Prchalová, E.; Kniežo, L.: An approach to stereoselective preparation of 3-C-glycosylated D- and L-glucals. Carbohydr. Res. 2010, 345, 352–362.
DOI: https://doi.org/10.1016/j.carres.2009.11.025
1. Parkan, K.; Vich, O.; Dvořáková, H.; Kniežo, L.: Stereoselective preparation of 2,3-dideoxy-3-C-[(α-D-galactopyranosyl)methyl]-D-arabino-hexopyranose and 2,3-dideoxy-3-C-[(α-D-galactopyranosyl)
methyl]-L-arabino-hexopyranose. Collect. Czech. Chem. Commun. 2008, 73, 690–700.
DOI: https://doi.org/10.1135/cccc20080690