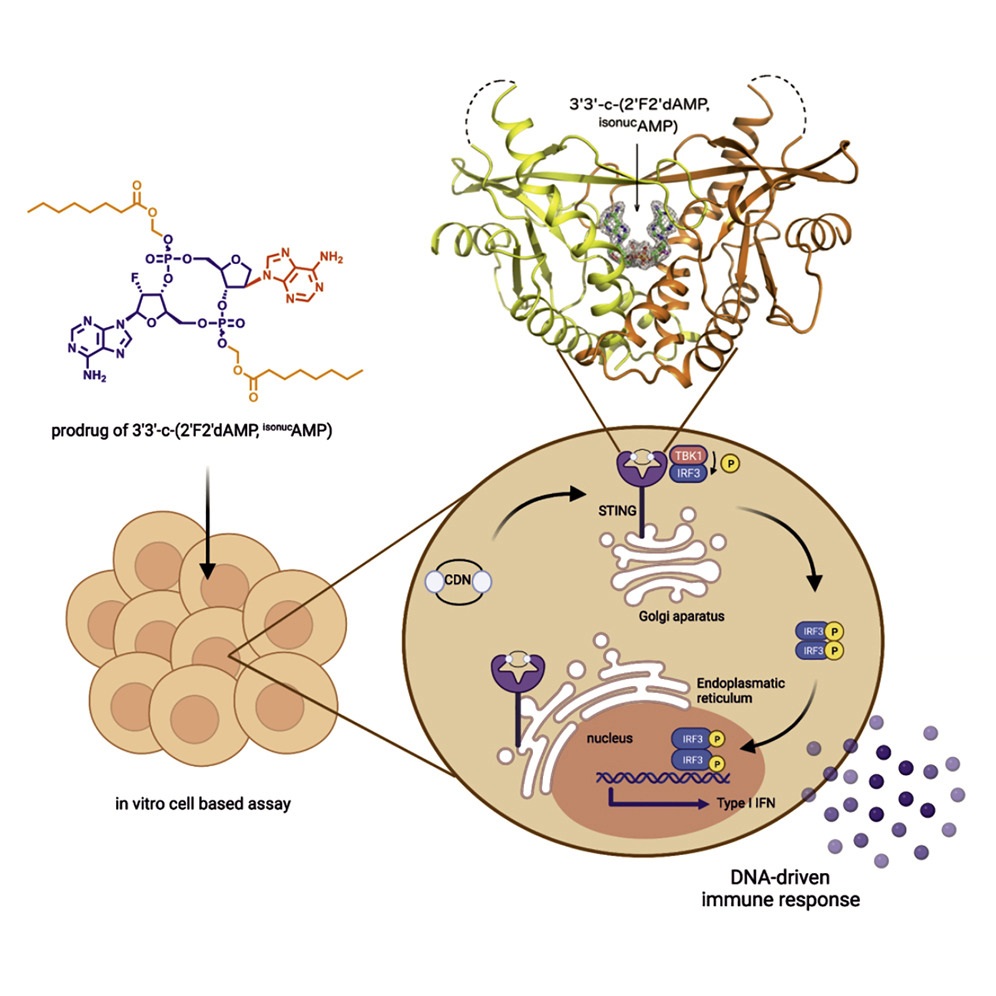
18. Dejmek M., Šála M., Brazdova A., Vanekova L., Smola M., Klíma M., Břehová P., Buděšínský M., Dračínský M., Procházková E., Zavřel M., Šimák O., Páv O., Boura E., Birkuš G., Nencka R.: Discovery of isonucleotidic CDNs as potent STING agonists with immunomodulatory potential. Structure 2022, 30(8), 1146-1156.
DOI: https://doi.org/10.1016/j.str.2022.05.012
17. Lášek T., Petrová M., Kosiová I., Šimák O., Buděšínský M., Kozák J., Snásel J., Vavrina Z., Birkus G., Rosenberg I., Páv O.: 5′-Phosphonate modified oligoadenylates as potent activators of human RNase L. Bioorg. Med. Chem. 2022, 56, 116632.
DOI: https://doi.org/10.1016/j.bmc.2022.116632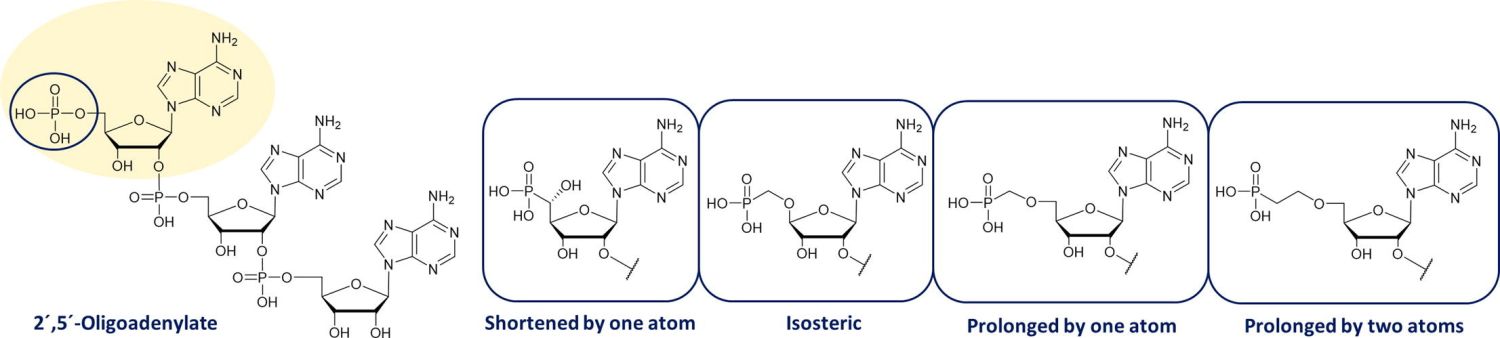
16. Pachl P., Šimák O., Buděšínský M., Brynda J., Rosenberg I., Řezáčová P.: Structure-based optimization of bisphosphonate nucleoside inhibitors of human 5(3)-deoxyribonucleotidases. Eur. J. Org. Chem. 2018, 37, 5144-5153.
DOI: https://doi.org/10.1002/ejoc.201800515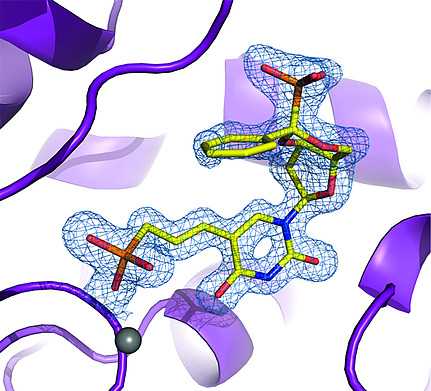
15. Seydlová G., Pohl R., Zborníková E., Ehn M., Šimák O., Panova N., Kolář M., Bogdanová K., Večeřová R., Fišer R., Šanderová H., Vítovská D., Sudzinová P., Pospíšil J., Benada O., Krížek T., Sedlák D., Bartůněk P., Krásný L., Rejman D.: Lipophosphonoxins II: Design, synthesis, and properties of novel broad spectrum antibacterial agents. J. Med. Chem. 2017, 60(14), 6098-6118.
DOI: https://doi.org/10.1021/acs.jmedchem.7b00355
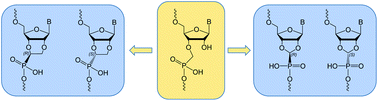
14. Páv O., Barvík I., Liboska R., Petrová M., Šimák O., Rosenbergová S., Novák P., Buděšínský M., Rosenberg I.: Tuning the hybridization properties of modified oligonucleotides: from flexible to conformationally constrained phosphonate internucleotide linkages. Org. Biomol. Chem. 2017, 15(3), 701-707.
DOI: https://doi.org/10.1039/C6OB02571D
13. Kostov O., Páv O., Buděšínský M., Liboska R., Šimák O., Petrová M., Novák P., Rosenberg I.: 4-Toluenesulfonyloxymethyl-(H)-phosphinate: A Reagent for the introduction of O- and S-methyl-(H)-phosphinate moieties. Org. Lett. 2016, 18(11), 2704-2707.
DOI: https://doi.org/10.1021/acs.orglett.6b01167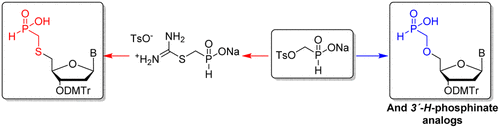
12. Panova N., Zborníková E., Šimák O., Pohl R., Kolář M., Bogdanová K., Večeřová R., Seydlová G., Fišer R., Hadravová R., Šanderová H., Vítovská D., Šiková M., Látal T., Lovecká P., Barvík I., Krásný L., Rejman D.: Insights into the mechanism of action of bactericidal lipophosphonoxins. PLoS ONE 2015, 10(12): e0145918.
DOI: https://doi.org/10.1371/journal.pone.0145918
11. Pachl P., Šimák O., Řezáčová P., Fábry M., Buděšínský M., Rosenberg I., Brynda J.: Structure-based design of a bisphosphonate 5'(3')-deoxyribonucleotidase inhibitor. MedChemComm. 2015, 6, 1635-1638.
DOI: https://doi.org/10.1039/C5MD00235D
10. Petrová M., Páv O., Buděšínský M., Zborníková E., Novák P., Rosenbergová Š., Pačes O., Liboska R., Dvořáková I., Šimák O., Rosenberg I.: Straightforward Synthesis of purine 4′-aAlkoxy-2′-deoxynucleosides: First report of mixed purine−pyrimidine 4′-alkoxyoligodeoxynucleotides as new RNA mimics. Org. Lett. 2015, 17, 3426-3429.
DOI: https://doi.org/10.1021/acs.orglett.5b01430
9. Šimák O., Pachl P., Fábry M., Buděšínský M., Jandušík T., Hnízda A., Skleničková R., Petrová M., Veverka V., Řezáčová P., Brynda J., Rosenberg I.: Conformationally constrained nucleoside phosphonic acids – potent inhibitors of human mitochondrial and cytosolic 5′(3′)-nucleotidases Org. Biomol. Chem. 2014, 12, 7971-7982.
DOI: https://doi.org/10.1039/C4OB01332H
8. Šípová H., Špringer T., Rejman D., Šimák O., Petrová M., Novák P., Rosenbergová Š., Páv O., Liboska R., Barvík I., Štěpánek J., Rosenberg I., Homola, J.: 5’-O-Methylphosphonate nucleic acids – new modified DNAs that increase the E. coli RNase H cleavage rate of hybrid duplexes. Nucleic Acids Res. 2014, 42, 5378-89.
DOI: 10.1093/nar/gku125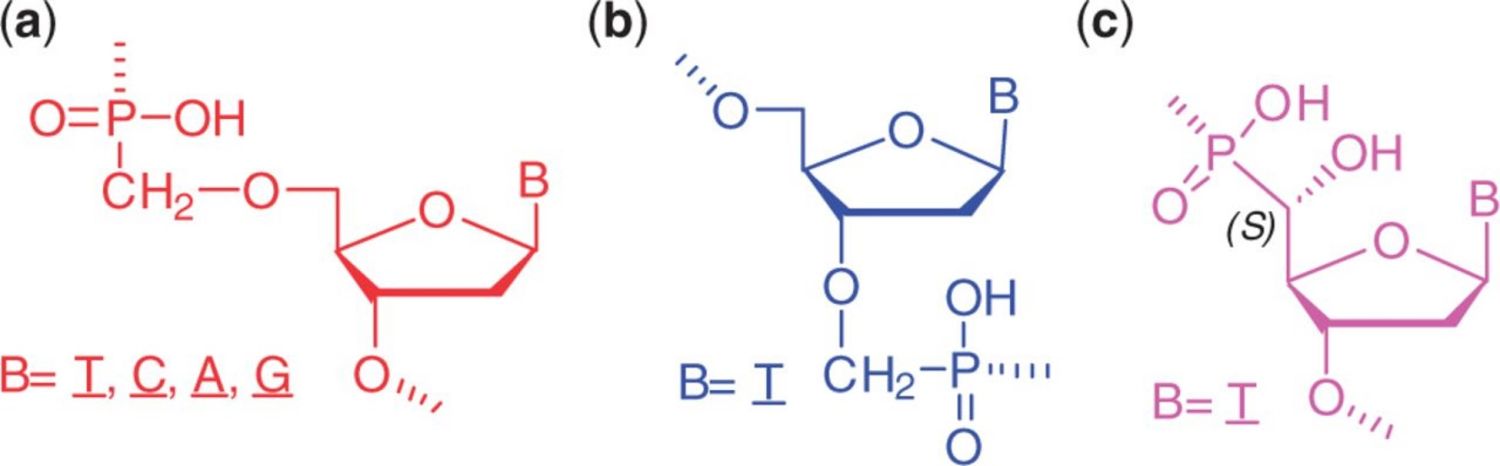
7. Šimák O., Košiová I., Panova N., Buděšínský M., Petrová M., Rejman D., Liboska R., Páv O., Rosenberg I.: Inhibition of human thymidine phosphorylase by conformationally constrained pyrimidine nucleoside phosphonic acids and their “open-structure” isosteres. Eur. J. Med. Chem. 2014, 74, 145-168.
DOI: https://doi.org/10.1016/j.ejmech.2013.12.026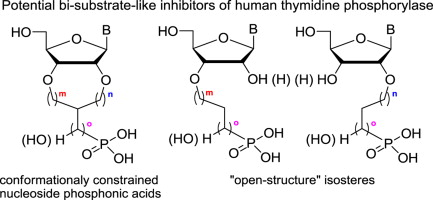
6. Pachl P., Fábry M., Rosenberg I., Šimák O., Řezáčová P., Brynda J.: Structures of human cytosolic and mitochondrial nucleotidases: implications for structure-based design of selective inhibitors. Acta Cryst. 2014, 70, 461-470.
DOI: https://doi.org/10.1107/S1399004713030502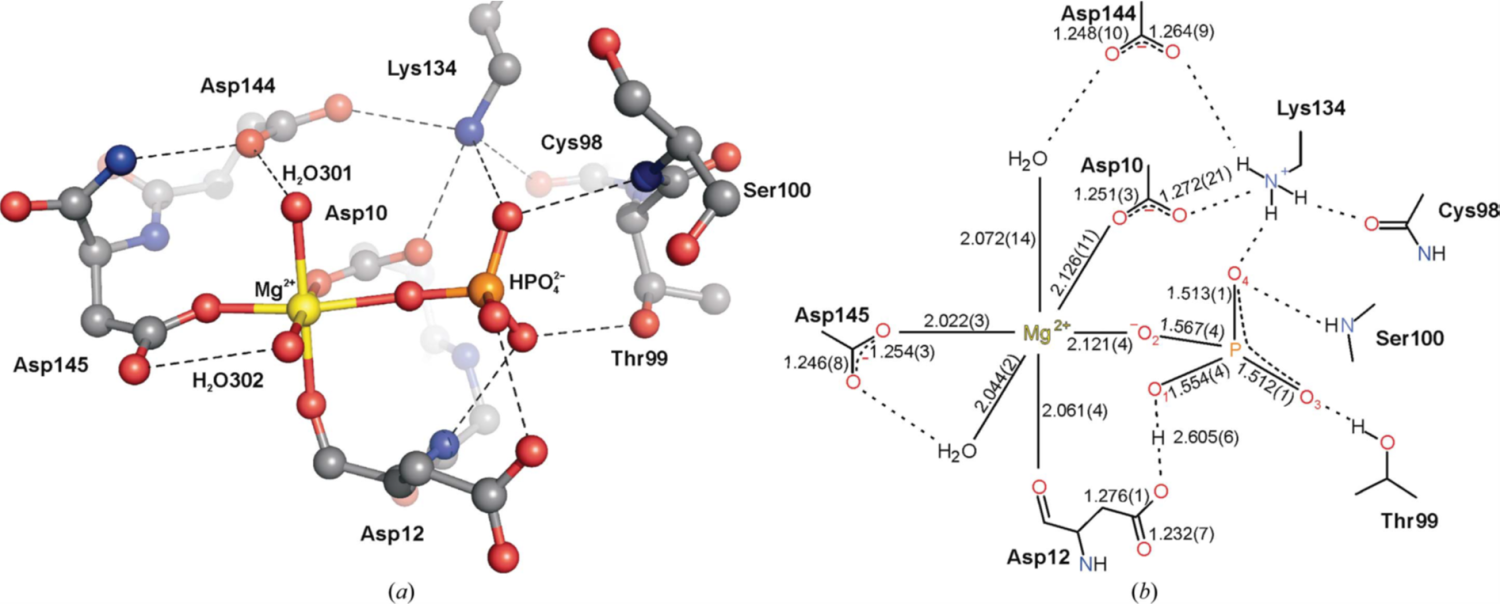
5. Zelenka K.; Trnka T., Tislerova I., Monti D., Cinti S., Naitana M.L., Schiaffino L., Vananzi M., Laguzzi G., Luvidi L., Mancini G., Nováková Z., Šimák O., Wimmer Z., Drašar P.: Spectroscopic, morphological, and mechanistic investigation of the solvent-promoted aggregation of porphyrins modified in meso-positions by glucosylated steroids. Chem. Eur. J. 2011, 17, 13743-13753.
DOI: https://doi.org/10.1002/chem.201101163
4. Štěpánek P., Šimák O., Nováková Z., Wimmer Z., Drašar P.: Asymmetrically substituted calix[4]pyrrole with chiral substituents. Org. Biomol. Chem. 2011, 9, 682-683.
DOI: https://doi.org/10.1039/C0OB00712A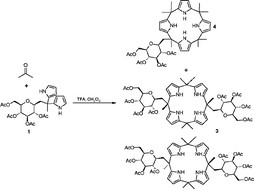
3. Pressová M., Buděšínský M., Košiová I., Kopecký V. (Jr.), Cvačka J., Kašička V., Šimák O., Točík Z., Rosenberg I.: Oligomerization of adenosin-5'-O-ylmethylphosphonate, an isopolar AMP analogue: Evaluation of the route to short oligoadenylates. Biopolymers 2010, 93, 277-289.
DOI: https://doi.org/10.1002/bip.21329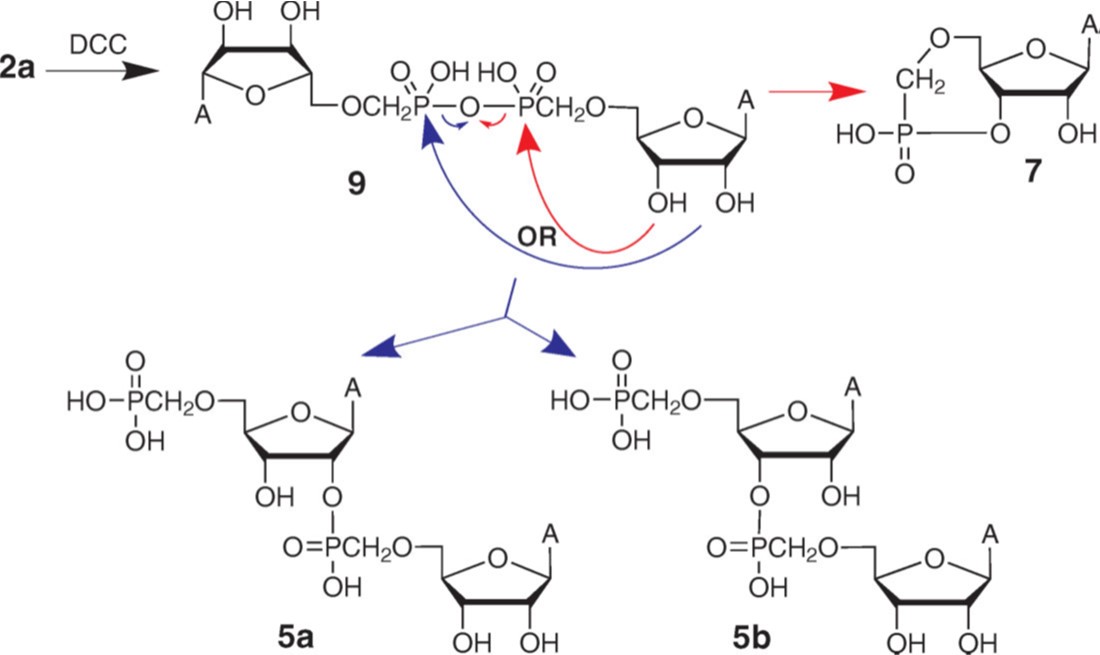
2. Košiová I., Točík Z., Buděšínský M., Šimák O., Liboska R., Rejman D., Pačes O., Rosenberg I.: Methyl 4-toluenesulfonyloxymethylphosphonate, a new and versatile reagent for the convenient synthesis of phosphonate-containing compounds. Tetrahedron Lett. 2009, 50, 6745-6747.
DOI: https://doi.org/10.1016/j.tetlet.2009.09.062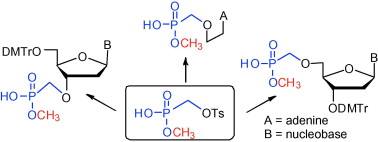
1. Šimák O., Staněk J., Moravcová J.: A stereocontrolled synthesis of 3-acetamido-1,3,5-trideoxy- and 1,3,5,6-tetradeoxy-1,5-imino-D-glucitol. Carbohydr. Res. 2009, 344, 966-971.
DOI: https://doi.org/10.1016/j.carres.2009.03.016
