Chemie nukleotidů a oligonukleotidů
Naše laboratoř je součástí skupiny Nukleotidů a oligonukleotidů na Ústavu organické chemie a biochemie AV ČR, v.v.i. Naše témata souvisí s prací této skupiny a vycházejí z ní. Leitmotivem naší práce je příprava fosfonových kyselin odvozených od nukleosidů. Látky tohoto typu již několikrát prokázaly své kvality - acyklické deriváty fosfonových kyselin odvozené od nukleosidů jsou účinnou složkou léků na HIV a jiné nebezpečné viry, bisfosfonáty jsou součástí léků proti osteoporóze.
V popředí našeho zájmu jsou oligonukleotidy, v nichž je fosfátová vazby mezi nukleotidovými jednotkami nahrazena vazbou fosfonátovou. Tato záměna esterové vazby P-O za enzymaticky neštěpitelnou vazbu P-C přináší do molekuly oligonukleotidu větší stálost v buňkách a tím zvyšuje jejich případný biologický účinek. Příprava oligonukleotidů probíhá na pevné fázi za využítí automatického syntetizátoru.
Během přípravy monomerů pro automatickou syntézu na pevné fázi připravujeme celou řadu strukturně rozmanitých derivátů nukleosidů, které ve spolupráci s dalšími pracovišti testujeme na jejich možné biologické účinky jak in vitro (enzymové testy), tak in vivo (testy na buňkách a buněčných liniích).
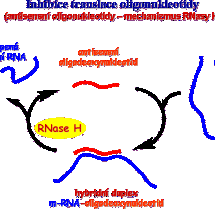
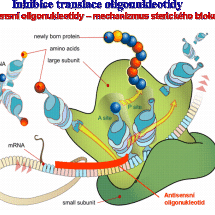
Námi připravené modifikované nukleotidy mohou ovlivnit replikaci DNA (ukončením růstu vznikajícího řetězce DNA) a oligonukleotidy složené s nukleotidových analogů mohou např. potlačit genovou expresi (mechanismus sterického bloku, RNAsa H) a ovlivnit tlumení genů (siRNA). Nukleosidfosfonové kyseliny však mohou také fungovat i jako inhibitory různých důležitých enzymů (thymidinfosforyláza, nukleotidázy) a ovlivnit tak dění ve zdravých i nemocných buňkách.
Studentům můžeme nabídnout účast v přátelském a vstřícném vědeckém kolektivu se značnými zkušenostmi v chemii nukleosidů, nukleotidů a oligonukleotidů, a to vše v zázemí modernizovaného ÚOCHB AV ČR.